Pfizer Fda Approval Age
11 hours agoThe FDA approved the two-dose vaccine for use in people age 16 and older. 13 hours agoSo Pfizer kept that study going and the FDA also examined real-world safety evidence in deciding whether to fully license the vaccine for people 16 and older those studied the longest.
11 for the Pfizer-BioNTech COVID-19 Vaccine for individuals 16 years of age and older was based on safety and effectiveness data from a randomized controlled blinded ongoing clinical trial of thousands of individuals.

Pfizer fda approval age. 11 hours agoIn its announcement the FDA said the Pfizer vaccine will be rebranded as ComirnatyIts been approved for anyone over the age of 16 and will retain emergency use authorization for minors 12 through 15 years of age and for giving a third booster shot to people who are immunocompromised. The Pfizer vaccine will now be marketed as Comirnaty for the prevention of. 9 hours agoPfizer and BioNTech have received the FDAs first formal approval for a COVID-19 vaccinethe messenger RNA mRNA-based BNT162b2 which according to the agency will be marketed going forward.
Acting FDA Commissioner Janet Woodcock called. 10 hours agoHealth officials hold a media briefing to discuss the FDAs approval of the first COVID-19 vaccine. 11 hours agoThe FDA gave emergency-use authorization to Pfizers vaccine for people age 16 and older in December - the first shot to gain such backing in the United States - and provided further emergency-use.
9 hours agoThe FDA approved the two-dose vaccine for use in people age 16 and older. On May 10 2021 the FDA expanded the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine to include adolescents 12 through 15 years of age. Food and Drug Administration on Monday gave full approval to the COVID-19 vaccine made by Pfizer Inc and German partner BioNTech SE for use in people over the age.
The emergency use authorization allows. 8 hours agoThe US. Food and Drug Administration approved the first COVID-19 vaccine for individuals 16 years of age and older.
The vaccine also continues to. Despite the Food and Drug Administrations full approval Monday of the Pfizer-BioNTech COVID-19 vaccine for ages 16 and older pediatricians are discouraging parents from trying to. 13 hours agoThe original trials mainly included adults and Covid-19 vaccine testing in younger children has only recently begun so the FDA has fully approved the PfizerBioNTech vaccine for.
The Food and Drug Administration has granted full approval to Pfizers COVID-19 vaccine for people age 16 and up the agency announced on Monday. Food and Drug Administration has approved the Pfizer-BioNTech COVID-19 vaccine for individuals 16 years of age and older. The Moderna and Johnson Johnson vaccines are still covered under an emergency use authorization as is.
11 2020 the Pfizer-BioNTech COVID-19 Vaccine has been available under EUA in individuals 16 years of age and older and the authorization was expanded to. The new official approval is for vaccine use in individuals who are 16 and older. 11 hours agoSince Dec.
Public health officials hope the action will convince more unvaccinated Americans that Pfizers. The FDA approval granted early today follows the emergency use authorization given to Pfizer last December. This is the first coronavirus vaccine approved by the FDA and is expected to open the door to more vaccine mandates.
Public health officials hope the action will convince more unvaccinated Americans that Pfizer s shot is safe and. 9 hours agoFDA Evaluation of Safety and Effectiveness Data for Approval for 16 Years of Age and Older The first EUA issued Dec. Pfizers COVID-19 vaccine is now fully approved for ages 16 and up the FDA announced today.

Fda Sets 2022 Deadline To Decide On Full Approval Of Pfizer Biontech Vaccine

Pfizer Submits Clinical Data On 3rd Dose To Fda For Authorization The Times Of Israel

Fda Grants Full Approval To Pfizer S Covid Vaccine

Pfizer Moderna To Expand Vaccine Trials In Children Ages 5 11 At Fda Request Report Says Wfla
Fda Gives Full Approval To Pfizer Vaccine World News Hindustan Times

Pfizer Says Fda Will Soon Authorize Covid 19 Vaccine For 12 15 Age Group Coronavirus Updates Npr

Pfizer Covid Vaccine Fda Clears Use In Kids Ages 12 To 15

Fda Oks Pfizer Covid 19 Vaccine For 12 15 Age Group Coronavirus Updates Npr

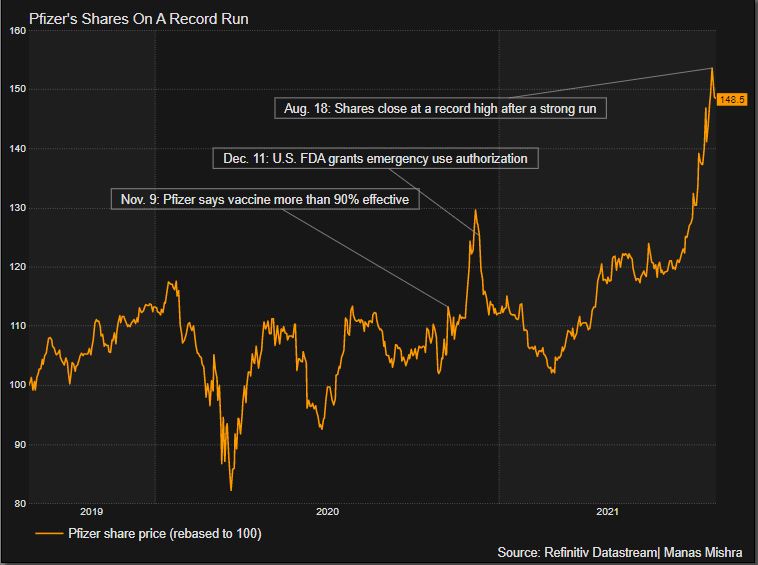


/cdn.vox-cdn.com/uploads/chorus_asset/file/22771059/1234462203.jpg)



Post a Comment for "Pfizer Fda Approval Age"